38 fda approved health claims on food labels
A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural." Qualified Health Claims | FDA Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
Decoding Food Label Claims: The Lowdown on Package Promises Free. Added to many food packages, the term "free" is regulated by the FDA and means the food lacks a perceived negative quality. Here are three examples: Fat-free: a food that contains less than 0.5 grams of fat per serving. Sugar-free: a food that has less than 0.5 grams of sugars per serving.

Fda approved health claims on food labels
Structure/Function Claims | FDA If a dietary supplement label includes such a claim, it must state in a "disclaimer" that FDA has not evaluated the claim. The disclaimer must also state that the dietary supplement product is not... Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,... 21 CFR § 101.14 - Health claims: general requirements. | CFR | US Law ... (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements (e.g., a brand name including a term such as "heart"), symbols (e.g., a heart symbol), or vignettes, characterizes the relationship of any substance …
Fda approved health claims on food labels. Is It Really 'FDA Approved'? May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ... Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim". ABC's of Health Claims - WebMD Calcium and osteoporosis. Dietary fats and cancer. Saturated fats and cholesterol and risk of coronary heart disease. Sugar alcohols and dental caries. Fiber and cancer. Folic acid and neural tube ... Qualified Health Claims: Letters of Enforcement Discretion | FDA Qualified Health Claims: Letters of Enforcement Discretion · Atopic Dermatitis · Cancer · Cardiovascular Disease · Cognitive Function · Diabetes · Hypertension.
Are the health claims on food labels accurate and reliable? The health claims must be balanced and based on current, reliable scientific studies and must be approved by the U.S. Food and Drug Administration (FDA). Health claims may be statements like "This food is a good source of calcium. FDA Label Claims For Low Carb Diets However, the U.S. Food and Drug Administration (FDA) regulate claims that are used on food and supplements labels, and it has categorized the applications into three categories as defined by the FDA statute: health claims, nutrient content claims, and structure/function claims. · Health claims: - These are statements that describe a ... Is Pfizer’s FDA-approved COMIRNATY Vaccine Available in the US? May 25, 2022 · On August 23, 2021, the US Food and Drug Administration (FDA) approved Pfizer’s biological licensing application (BLA) for its covid-19 vaccine named COMIRNATY for people aged 16yrs and older. At the time, vaccine hesitancy was persistent and the acting FDA Commissioner Janet Woodcock said that granting full approval to the vaccine might ... Food Packaging Claims | American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims.
Food and Drug Administration - Wikipedia The United States Food and Drug Administration (FDA or USFDA) is a federal agency of the Department of Health and Human Services.The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood ... How to Get FDA Approval | Registrar Labeling FDA Approved Products. Manufacturers of drugs and devices that do require FDA approval may include the phrase “FDA Approved” on the product’s labeling, as long as the manufacturer has received a letter from FDA confirming its approval. The FDA logo should not be used on a product’s labeling whether the product is approved or not. Food Labeling: Health Claims; Dietary Guidance - Federal Register 1. The NLEA authorized health claims in food labeling by amending the Federal Food, Drug, and Cosmetic Act (the act) to add section 403(r) to the act (21 U.S.C. 343(r)). This section specifies, in part, that a food is misbranded if it bears a claim that expressly or by implication characterizes the relationship of a nutrient to a disease or ... Use of the Term Healthy on Food Labeling | FDA 25 Mar 2022 — While FDA is considering how to redefine the term "healthy" as a nutrient content claim, food manufacturers can continue to use the term " ...
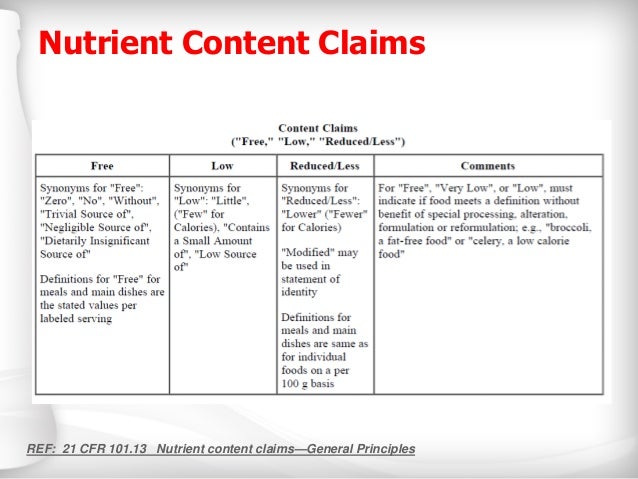
Nutrient Content Claims | FDA Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ...
Factual Food Labels: Health Claims - University of Texas at Austin According to the United States Food and Drug Administration (FDA) there are only three categories of claims that are approved to be printed on food packaging: health claims, nutrient claims, and function claims. Generally, these labels are found on the front side of the food package in emphasized lettering. Health Claims
What are some examples of an FDA health claim on a food label? Similarly, what health claims are allowed on food labels? Approved Health Claims Calcium, Vitamin D, and Osteoporosis. Dietary Lipids (Fat) and Cancer. Dietary Saturated Fat and Cholesterol and Risk of Coronary Heart Disease. Dietary Non-cariogenic Carbohydrate Sweeteners and Dental Caries.
Health Claims on Food Labels | LegalMatch Health claims must be approved by the Food and Drug Administration (FDA) before the manufacturer is allowed to put the claim on one of their food products . There are two ways of obtaining FDA approval: First, the manufacturer can come up with a health claim based on independent scientific studies and evidence.
Questions and Answers on Health Claims in Food Labeling All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if...
FDA approves cardiovascular health claim on certain oil labels ... Regardless, the United States Food and Drug Administration has announced that it will allow all olive oil bottles to carry a new "qualified health claim" on their labels. With this, manufacturers...
Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.14 Health claims: general requirements. (a) Definitions. For purposes of this section, the following definitions apply: (1) Health claim means any claim made on the label or in labeling of a food, including a dietary supplement ...
Food labelling and packaging: Nutrition, health claims and ... - GOV.UK Nutrition labelling. You must follow nutrition labelling information rules for all pre-packed products unless both of the following apply: you're a small business with under 10 employees and a ...
ch2 Flashcards | Quizlet health claim on a food label that has been approved based on emerging but not well-established evidence of a relationship between a food, food component, or dietary supplement and reduced risk of a disease or health-related condition; must be accompanied by an explanatory statement to avoid misleading customers
Protein Label - LabelCalc LabelCalc's online database analysis will give you accurate FDA-compliant food labels with FDA-Approved Health Claims included. LabelCalc is an industry-leading recipe analysis tool used by food manufactures, global retail stores and food entrepreneurs. To get started, see our pricing today.

34 Which Of The Following Claims Could Not Appear On A Supplement Label Without Fda Approval ...
health claims and food labels Flashcards | Quizlet It is a claim on a food product that directly or by implication characterizes the level of a nutrient in the food etc: low (lo), high (hi), free, without, no, zero structure function claims -role of a nutrient in humans -can't mention a disease -"calcium builds strong bones" supplements must say
Health Claims On Your Food - Tufts Health & Nutrition Letter Although some people may call any type of health or nutrition message on a food package a "health claim," this isn't really consistent with the FDA's definition. Packaged food and beverage labels may carry four general types of claims, which include health claims, qualified health claims, structure/function claims and nutrient content claims.
Authorized Health Claims That Meet the Significant Scientific Agreement ... Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
Health Claims on Food Labels: LabelCalc - FDA Compliant Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified.". Authorized Health Claims: Claims that have significant scientific agreement (SSA).
FDA Label Search Search for Labels on DailyMed. The labels are also available on the National Library of Medicine's DailyMed. 4. web site. You can search for labels by drug name and link to the Library's information resources about marketed drugs.
Nutrient Claims on Food Labels - Clemson University Lean Claims. Lean. Contains less than 10 grams total fat, 4.5 grams or less saturated fat, and less than 95 milligrams cholesterol. Extra lean. Contains less than 5 grams total fat, less than 2 grams saturated fat, and less than 95 milligrams cholesterol. *compared to the reference, or regular, food this would replace.

![Food Labeling 101 - FDA Regulations Guide [2021] | Artwork Flow](https://uploads-ssl.webflow.com/5f59aa263c234bb74025de57/5fa4f8a355c6935dd2dde09d_Inner-Images-1.jpg)


Post a Comment for "38 fda approved health claims on food labels"